The research endeavours of CLASSY focus on beyond state of the art advances in three main areas:
Programmable replicating peptide catalysis
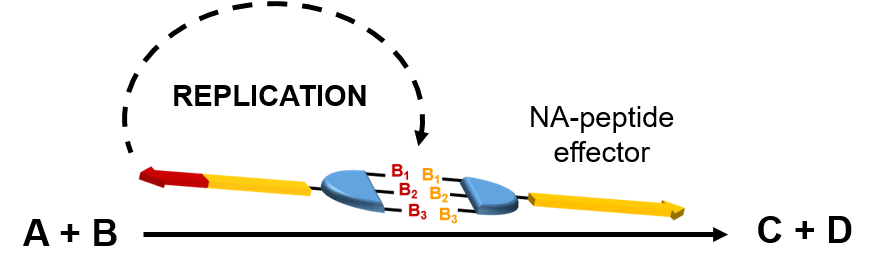
Preliminary results from the Ashkenasy and de la Escosura labs have revealed that it is possible to control the replication process of NA–peptide hybrids through complementary base-pairing interactions the same way in which DNA stores genetic information. The challenge is to impart catalytic activity to these NA– peptide hybrids. The overall idea is to programme catalytic activity in a genetic-like manner, through informationally controlled self-synthesising catalysts. This breakthrough is far beyond the present possibilities of catalytic technology and will offer great advantages for the selectivity of chemical transformations. In a further step, this concept will also be applied to cascade reactions. For example, in a model target cascade we would ideally initiate the first step, a cross aldol reaction, controlling the catalyst activity within a replication network, thus triggering from that moment the whole cascade process.
Compartmentalisation of catalytic replication networks
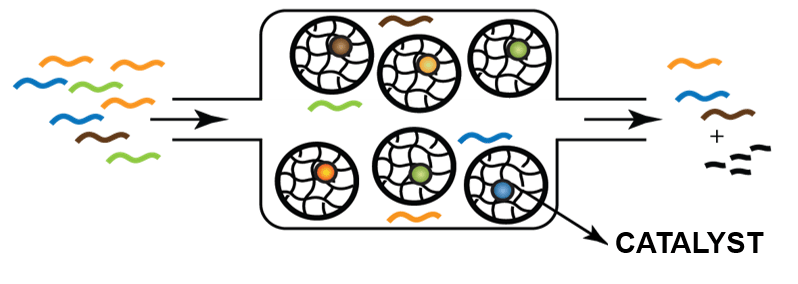
Compartmentalisation of catalytic replication networks offers advantages such as the separation of incompatible reactions into different microchambers and control of the catalytic replication network through molecular crowding. A critical issue here is to perform high-throughput screening of the resulting microreactors through many different combinations of building blocks. We will use robust microfluidic sorting and analysis methods to isolate the compartments with favourable combinations of functional building blocks from non-functional ones (e.g. through on-chip fluorescence and off-chip MS detection techniques). These compartments are expected to enhance the catalytic performance of replicating catalysts, which will open a completely new perspective for the application of systems chemistry in chemical synthesis.
Reaction sequences occurring in microfluidic molecular assembly lines
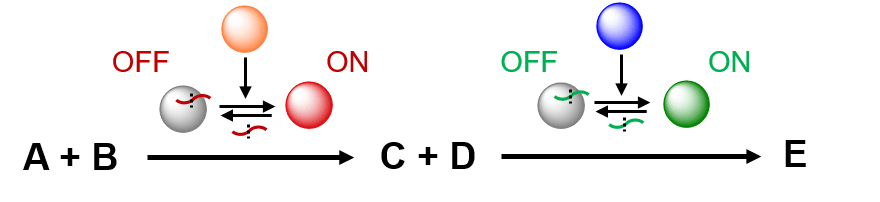
As enzymes offer a much wider toolbox of cata- lysts than peptides, we also plan to compartmentalise enzymatic cascade processes in microfluidic hydrogel beads. The Huck group has recently developed a novel method to control the activity of proteases using reversible peptide inhibitors. The key principle of this approach is that enzymatic activity can be switched ON or OFF by reversibly binding peptides, which can be cleaved by the same or other enzymes. In this way, a ‘chain’ of enzymes can be formed, controlling each other’s activity. In CLASSY, we will expand this approach by incorporating enzymes with more useful catalytic functionalities (e.g. oxidoreductase, dehydrogenase, dehalogenase, decarboxylase), which will enable controlled programming of reaction sequences in a cell-like molecular assembly line.
New advances in systems chemistry and chemical network
The project coordinator, Prof. Andrés de la Escosura (Universidad Autónoma de Madrid, Spain), and Prof. Gonen Ashkenasy (Ben-Gurion University of the Negev, Israel) achieved new insights into the dynamic self-organisation of multi-component synthetic systems. At the same time, the groups of Prof. Helma Wennemers (ETH Zürich, Switzerland) and Prof. Wolfgang Kroutil (Universität Graz, Austria) joined mutual efforts to investigate the efficiency of enzymes in the presence of peptide catalysts. The Wennemers group has successfully demonstrated the use of peptides as efficient catalysts that are robust and versatile in applications and together with the group of Prof. Kroutil they were able to show that a combination of peptide- and biocatalysts in a single pot-reaction, can complete a two-step biocatalytic process. The work of Prof. Wilhelm Huck (Radboud University, Netherlands) instead focused on the study of chemical reaction networks and led research efforts on microfluidic production of catalytic reactors, supported by Micronit B.V., a company based in Netherlands specialised in the development of microfluidic chips.
Research highlights and wide-ranging collaborationS
The most recent CLASSY findings were highlighted in the project’s final Scientific Symposium event—“Catalysis in chemical networks and supramolecular assemblies: New advances in systems chemistry”—held online on 15 March 2024 and attracting over 50 participants from 42 organisations. The Symposium was key in disseminating the outcomes of the project both within the consortium and to external participants from academic institutions and research companies. The partners had the opportunity to share their latest discoveries with a wider audience on the topics with the view of four external experts in catalysis and compartmentalisation strategies in the context of systems chemistry. All members also shared insights on the importance of facing challenges to revolutionise the methodologies in which we synthesise chemical molecules. The event marked a successful end to this ambitious and multidisciplinary project, achieving not only significant develops in the fundamental understanding of molecular synthesis, but also strong collaborations between internal and external partners of the CLASSY consortium.
